|
| Dr. Alex Carlisle |
Dr. Alex
Carlisle is a neuroscientist in the Department of Neurosciences at Inova
Fairfax Hospital and head of the Laboratory of Neuro-Oncology at Krasnow. He is
a cancer biologist who uses molecular-based approaches to identify and
functionally characterize molecules involved in the progression of various
cancers. On December 6th, 2012, he presented a power point
presentation to our NEUR410 course discussing his current research: Biological
and Clinical Association of CXCR4 with the Development of Peripheral
Neuroblastic Tumors (pNTs).
Neuroblastoma is a disease where malignant (cancerous) tumors start to develop in the sympathetic nervous system and may spread to all
other parts of the body. It mainly affects infants and children. The cause of
this tumor is currently unknown but Dr. Carlisle explained Phox2B, a gene found
in the development of sympathoadrenal cell lineage, may be an indicator of this
disease.
 |
| Neuroblastoma is a disease in which malignant (cancer) cells form in nerve tissue of the adrenal gland, neck, chest, or spinal cord. |
Neuroblastoma is the number one cancer found in infants,
accounting for approximately 10% of all pediatric cancers and 15% of cancer
related deaths in children. Early detection is vital but too often when
patients are first diagnosed, it has already spread. Children who are diagnosed
at 1 year old or less have the best possible outcome. The severity of their
diagnosis ranges from Stage I (isolated cancer) to Stage IV (metastasis). Studies have shown on rare occasion, stage IV neuroblastoma sometimes undergoes spontaneous regression without therapeutic intervention. “We don’t know why this happens”, said Carlisle. Treatments include surgery, chemotherapy, and/or radiation. Statistics show more powerful
biomarkers and improved therapies are urgently needed to diagnose
neuroblastoma. What role does Carlisle’s research
play in all this?
The Carlisle laboratory studies how molecules in tumor cells
from patients interact to promote the aggressive behavior of neuroblastoma. This
approach allows Carlisle to identify biomarkers which may aid in improving diagnosis
and prognosis. The heterogeneity of this disease makes is very difficult to
study but Carlisle has identified a chemokine receptor, CXCR4, whose expression
is clinically correlated with advanced stages of neuroblastoma.
 |
| Types of Neuroblastoma |
The biological roles for CXCR4 include inflammation
(lymphocyte homing and recruitment into inflammatory sites), neuronal
development (NPC migration during embryogenesis), metastasis, HIV infection (co-receptor
for HIV binding and fusion; CD4+ cells), and cancer progression. Would knocking
out CXCR4 solve the problem? No, CXCR4 is necessary for normal neuronal
formation and development of an organism. A genetic knockout would yield growth
malformation of the dorsal root ganglia which would kill the organism.
 |
| CT image of neuroblastoma tumor |
In an attempt to draw correlations with transcription
factors, Carlisle found little evidence to show there was any significance. Do
CXCR4 and MYCN interact? MYCN is a gene associated with a variety of tumors,
most notably neuroblastomas. I would assume an amplification of this gene would
present with higher levels of CXCR4 and neuroblastomas but Carlisle’s data
showed no correlation between the two.
Despite numerous roadblocks, Carlisle was able to find a
successful treatment for these cancer cells while evaluating CXCR4-mediated
signaling pathways associated with neuroblastoma progression. Plerixafor blocks
CXCR4 (receptors for only CXCL12) and as a result, inhibits growth and migration
of cancer cell and tumor growth. It is presumed this is accomplished by
preventing macrophages from being recruited to tumors.
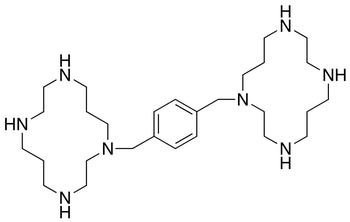 |
| Chemical structure of Plerixafor |